CER mechanism of action
Chimeric engulfment receptors (CERs) are unique, genetically engineered, multifunctional proteins comprising extracellular, transmembrane, and intracellular domains that are linked to induce target-specific anti-tumor effects.
The CER construct is designed to be multifunctional
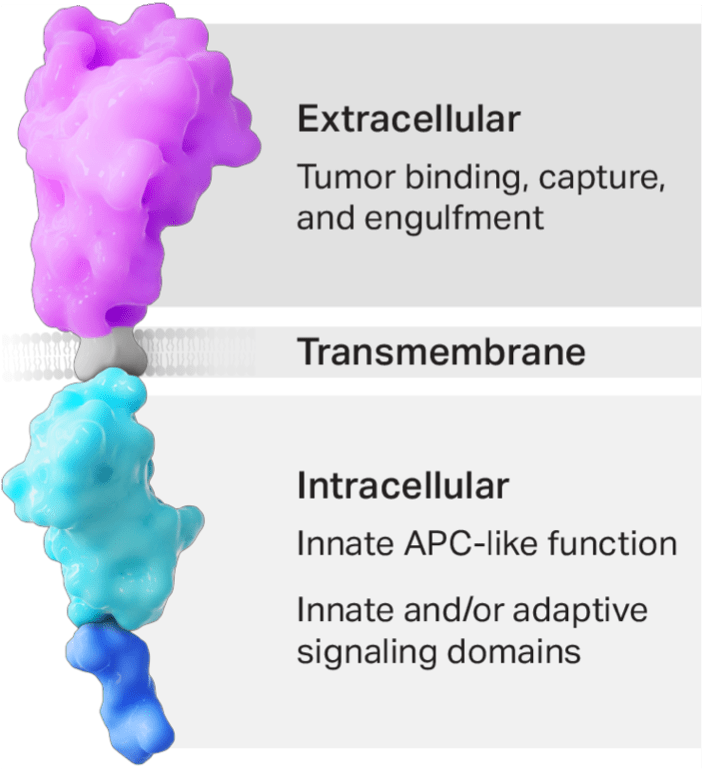
- The CER T cell’s phagocyte receptor component binds to the eat me signal on tumor cells.
- Binding mediates phagocytic uptake.
- The transmembrane domain links the receptor to intracellular domains.
- The APC-like intracellular domain allows for enhanced processing and presentation of tumor-specific antigens, which is expected to amplify the body’s endogenous adaptive T-cell response against the tumor.
- The intracellular signaling domain(s) activate T-cells, leading to cytotoxic killing of the targeted tumor.
First-generation CER T cells target eat me ligands upregulated on tumor cells
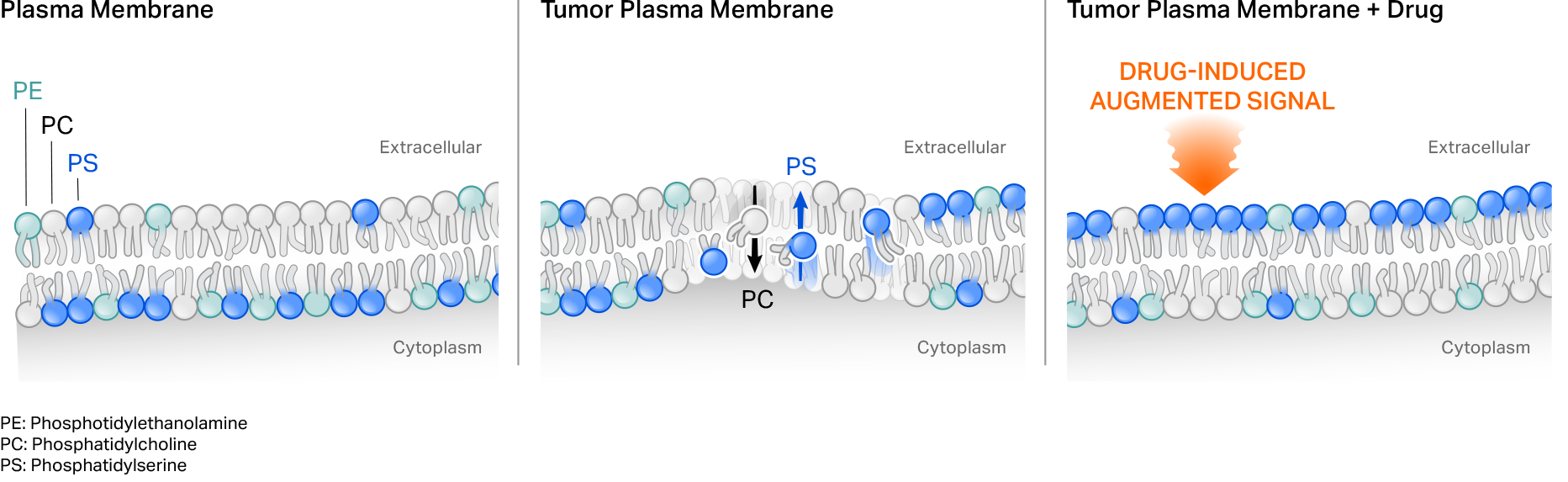
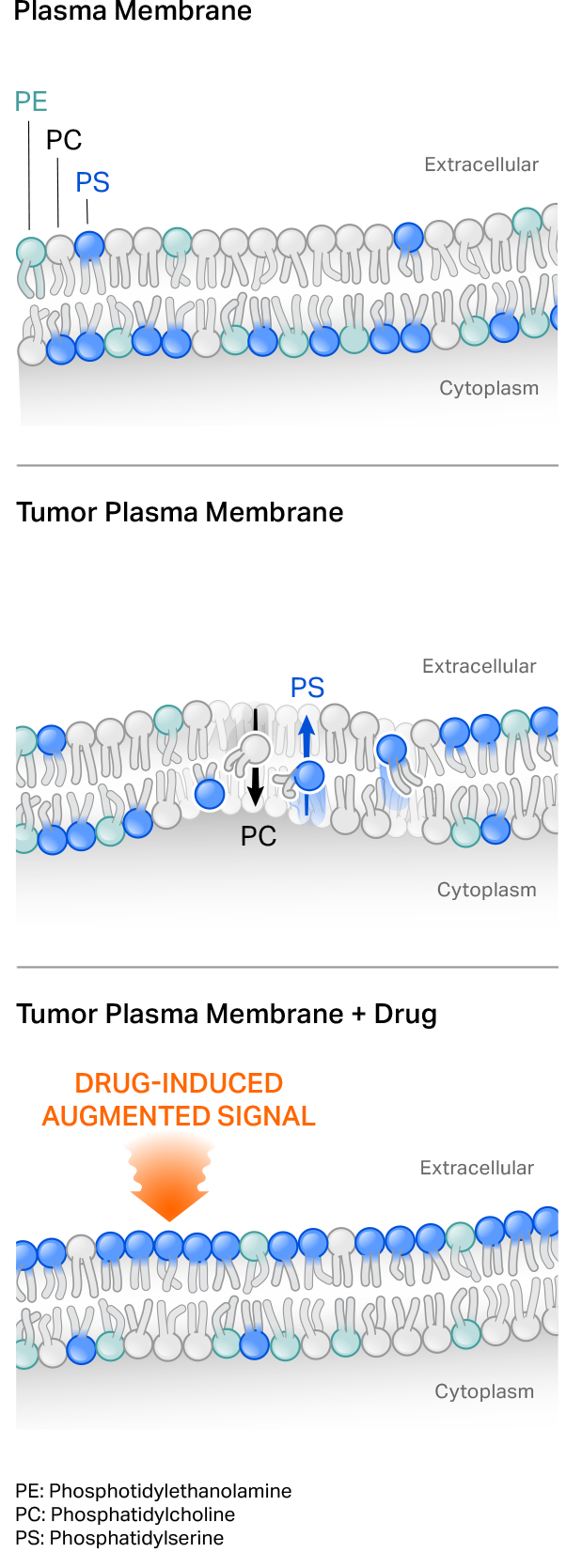
Our first-generation CER T cells employ a naturally occurring phagocyte receptor that binds to the phospholipid phosphatidylserine (PS) externalized on the surface of tumor cells. PS normally is present on the inside of a cell’s plasma membrane, but shifts to the external leaflet in pathologic settings such as in transformed or virally infected cells, or under stress conditions. Tumor-targeting cytotoxic agents can also further augment target on tumor cells to potentially drive CER T cell function.
CER T cells are designed to have a dual mechanism of action
CER-T cells function at the interface of the innate and adaptive immune systems, making them uniquely suited for cancer immunotherapy. Binding of the CER induces phagocyte-like engulfment activity of the CER T. The intracellular domains of the CER have the capacity to induce the complementary effects of
- Direct tumor killing mediated by CER T cells
- Secondary endogenous immune responses through APC-like presentation of tumor-associated antigens
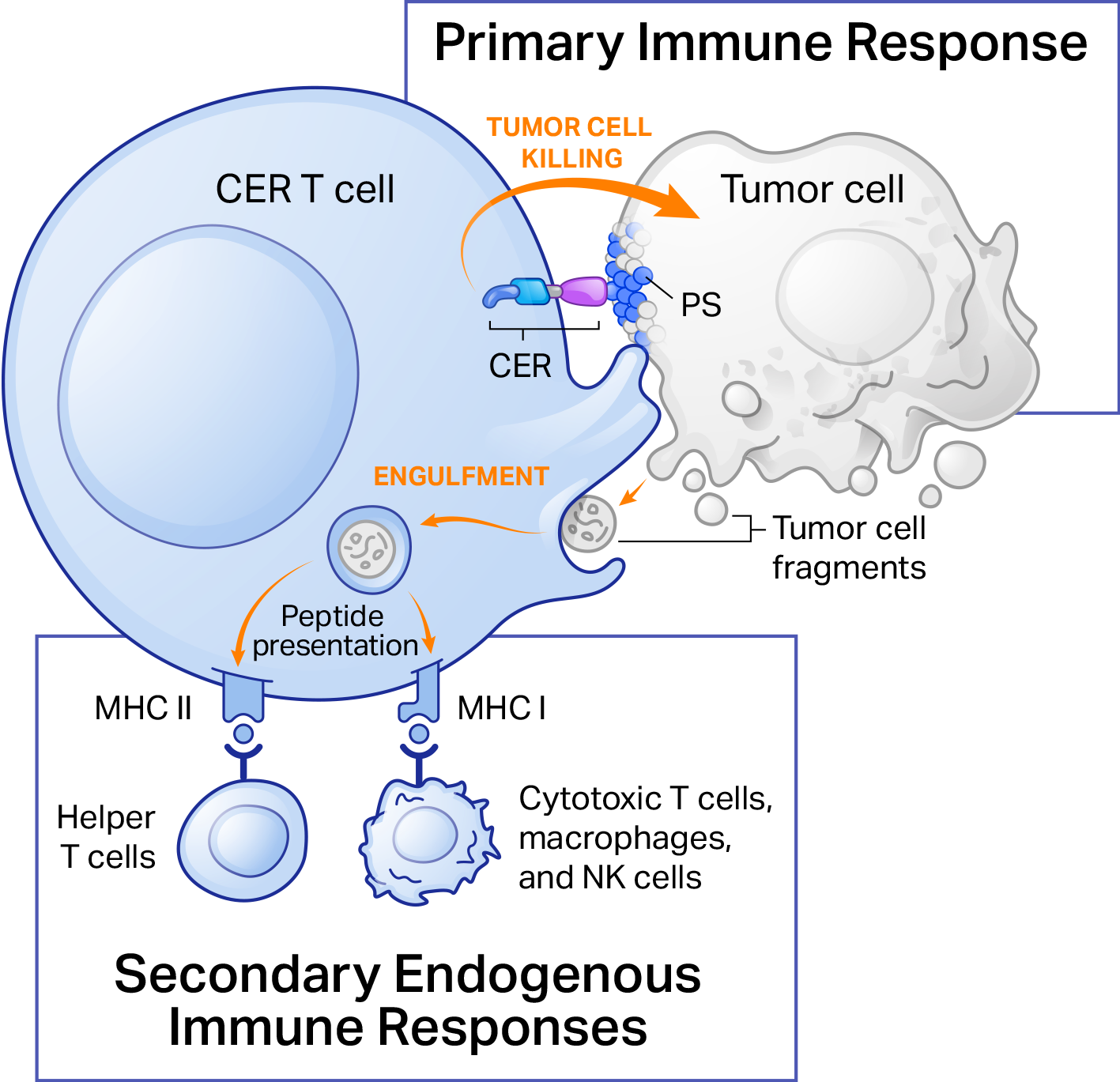
Thus, CERo’s technology has the potential to overcome major barriers to successful adoptive cell therapy by combining direct cell killing and phagocytic antigen presentation into single T cells to achieve tumor control.
With respect to tumor-associated barriers, eat me signals, unlike protein antigens, are not under genetic control. Thus, tumor heterogeneity in terms of target expression, which can lead to escape of target-negative cells, is unlikely to be a potential cause of relapse with CER T-cell therapy.
CER T cells also have the potential to overcome tumor microenvironmental immune suppression. One mechanism by which tumor cells escape immune surveillance is by disabling the process of tumor antigen uptake, processing, and presentation, resulting in poor cytotoxic anti-tumor immune responses. The additional APC-like activity of CER T cells has the capacity to prime tumor-specific cytotoxic T cells, leading to a robust secondary immune response sufficient for tumor eradication. Indeed, studies have shown that infiltration of T cells, especially CD8 T cells into tumor microenvironment, correlates with better prognosis in multiple malignancies such as lymphoma, breast, lung, melanoma, colorectal, and brain cancer.